Health October 26, 2025
Oxymetazoline Hydrochloride and Pregnancy: Essential Safety Guide
When it comes to clearing a stuffy nose, many people reach for a quick spray. But if you’re expecting a baby, you might wonder whether oxymetazoline pregnancy is safe. This guide walks you through what the drug does, how it behaves in a pregnant body, and what doctors recommend.
Understanding Oxymetazoline Hydrochloride
When it comes to nasal relief, Oxymetazoline Hydrochloride is a topical alpha‑adrenergic agonist used in over‑the‑counter nasal sprays to shrink swollen blood vessels in the nasal passages. By tightening the vessels, it reduces swelling and opens up airflow within minutes. The effect lasts up to 12 hours, which is why the product is popular for colds, allergies, or travel‑related congestion.
How Pregnancy Changes Drug Safety
Pregnancy introduces two new considerations for any medication: the mother’s altered physiology and the developing fetus. Blood volume rises, renal clearance speeds up, and the placenta becomes a semi‑permeable barrier. Some drugs cross the placenta easily, while others are blocked. The key question is whether oxymetazoline reaches the fetus in amounts that could cause harm.
Pharmacokinetics of Oxymetazoline in Expectant Mothers
Oxymetazoline is administered as a spray, and only a tiny fraction is absorbed systemically. Studies in healthy adults show plasma concentrations well below 0.1 µg/L after a single dose. During pregnancy, the same low absorption is expected because the drug remains largely confined to the nasal mucosa. There is no robust evidence that the compound crosses the placenta in meaningful amounts, but data are limited.
Regulatory Classification
Both the U.S. FDA and the European Medicines Agency (EMA) place oxymetazoline in a category that reflects insufficient human data for pregnancy. In the older FDA system it was labeled Category C, meaning animal studies have shown some risk but no well‑controlled studies in pregnant women exist. The newer FDA labeling requires a narrative risk summary instead of letters. The EMA calls it “use only if the benefit outweighs the risk.”
| Drug | Regulatory Category (US) | Regulatory Category (EU) | Typical Recommendation |
|---|---|---|---|
| Oxymetazoline | Category C (old); Narrative Risk | Use with caution | Short‑term, limit to 3‑day use |
| Phenylephrine | Category C | Use with caution | Same limits as oxymetazoline |
| Saline spray | Category A (safe) | Category A | Safe for unrestricted use |
Potential Maternal Side Effects
Even though systemic exposure is low, oxymetazoline can cause local side effects: burning, dryness, or a temporary runny nose after the spray wears off. Rarely, overuse leads to rebound congestion (rhinitis medicamentosa), where blood vessels become dependent on the drug and swell more once you stop. Pregnant users should avoid using the spray for more than three consecutive days.
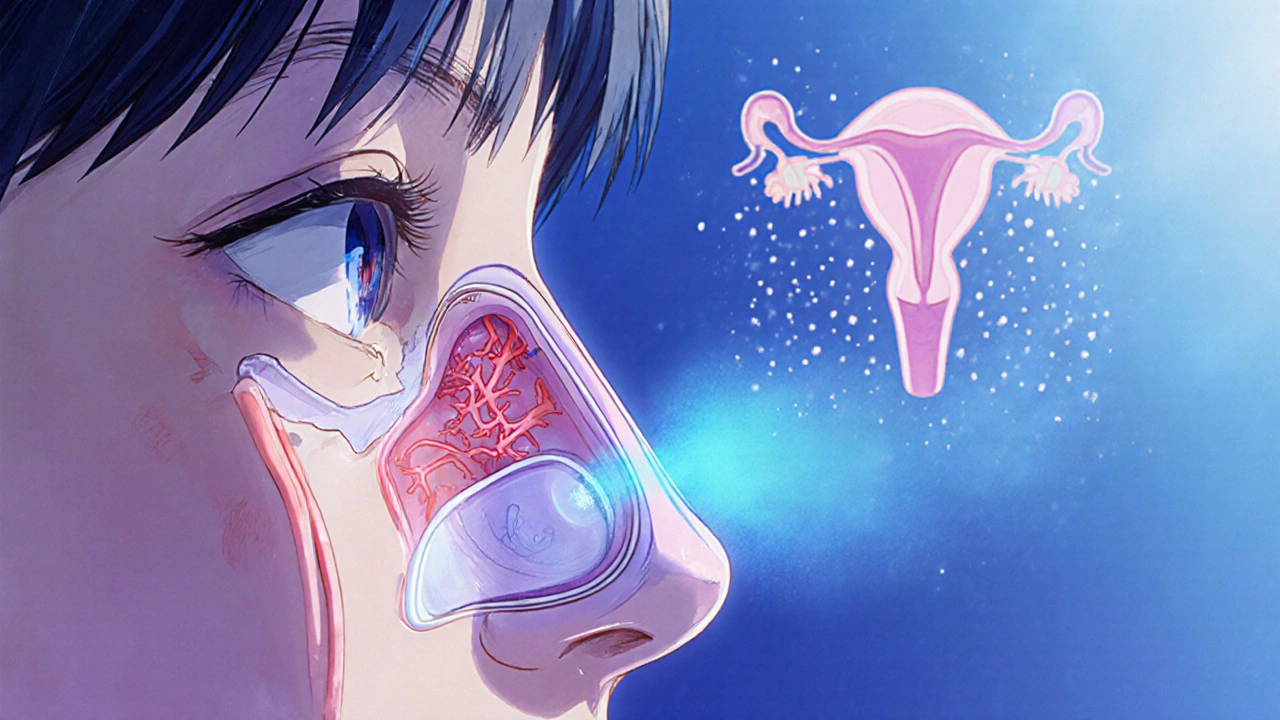
Fetal Concerns: What We Know
Animal studies in rats have shown high doses can cause reduced fetal weight and occasional skeletal variations. However, those doses are many times higher than the amount a human would absorb from a nasal spray. No human case reports link occasional oxymetazoline use to birth defects. The main theoretical risk is vasoconstriction that could reduce uterine blood flow, but the low systemic level makes this unlikely.
Guidelines from Professional Bodies
The UK’s National Institute for Health and Care Excellence (NICE) does not list oxymetazoline as a first‑line option for pregnant patients. Instead, they recommend saline sprays or steam inhalation for mild congestion. If a decongestant is truly needed, NICE suggests discussing the risk‑benefit balance with an obstetrician and limiting use to the shortest possible period.
Practical Tips for Expectant Mothers
- Start with a saline spray. If that isn’t enough, consider a single dose of oxymetazoline and see how you feel.
- Limit use to no more than three days in a row. Take a break of at least 24 hours before another short course.
- Read the label carefully for concentration; most OTC products contain 0.05 % oxymetazoline.
- Stay hydrated and use a humidifier; moisture helps thin mucus and reduces the urge to spray.
- Talk to your midwife or obstetrician if you have chronic sinus issues; they may prescribe a nasal corticosteroid that is safer in pregnancy.
When to Seek Medical Advice
If congestion lasts longer than a week, or is accompanied by fever, facial pain, or thick yellow discharge, you could have sinusitis, which may need antibiotics. In pregnancy, doctors prefer narrow‑spectrum antibiotics that are proven safe. Never self‑diagnose a bacterial infection; over‑the‑counter sprays won’t treat it.
Alternatives to Oxymetazoline
Besides saline, other options include:
- Hydration and steam inhalation - simple but effective.
- Nasal corticosteroid sprays such as budesonide - low systemic absorption and categorized as safe for pregnancy.
- Menthol rubs - provide a subjective feeling of easier breathing.
Bottom Line
Oxymetazoline Hydrochloride is a powerful short‑term nasal decongestant. In pregnancy, the limited systemic absorption means the fetal risk is low, but the lack of large human studies keeps it in a cautious category. Use it only when absolutely needed, keep each course under three days, and always discuss with your healthcare provider. Safer alternatives exist, so reserve oxymetazoline for moments when other measures fail.
Can I use oxymetazoline throughout my whole pregnancy?
No. Because safety data are limited, health authorities recommend only short‑term use (no more than three consecutive days) and only when other options don’t work.
Is a saline nasal spray safe?
Yes. Saline sprays contain only water and salt, making them safe for unrestricted use during pregnancy.
What are the signs of rebound congestion?
If your nose feels more blocked after stopping the spray, or if you need to use it more frequently, you may be experiencing rhinitis medicamentosa. Stop the spray and switch to saline or a steroid spray.
Are there any approved decongestants for pregnancy?
Officially, no oral decongestants are approved for pregnant women because of systemic effects. Topical options like oxymetazoline are allowed only with caution; saline and steroid sprays are preferred.
Should I talk to my midwife before using any nasal spray?
Yes. Even over‑the‑counter products can have subtle effects. Your midwife can help you weigh the benefits against potential risks and suggest the safest alternative.
Write a comment
Items marked with * are required.





11 Comments
Kasey Marshall October 26, 2025 AT 13:16
Oxymetazoline works locally so only a tiny amount gets into the bloodstream which makes it generally safe for short term use in pregnancy
Dave Sykes November 1, 2025 AT 13:26
If you’re dealing with a stuffy nose during pregnancy try a saline spray first then, if needed, a single dose of oxymetazoline-keep it under three days and you’ll stay on the safe side
Erik Redli November 8, 2025 AT 12:06
Everything you’re reading is just a smear campaign-oxymetazoline is a proven vasoconstrictor and there’s no solid data showing any risk to the fetus, so the “caution” tag is overblown
Jay Campbell November 15, 2025 AT 10:46
I’d add that many OB‑GYNs actually prescribe short courses when other measures fail, so it’s not unheard of to use it responsibly
Laura Hibbard November 22, 2025 AT 09:26
Wow, because a tiny spray is totally a “big danger” 🙄
Rachel Zack November 29, 2025 AT 08:06
People shouldnt ignore the fact that using unproven drugs while pregnant is just irresponsable and shows a lack of respect for the unborn child
Lori Brown December 6, 2025 AT 06:46
Hey Erik, I get the point but it’s still wise to follow the three‑day rule 😊 the benefits usually outweigh the tiny theoretical risk when you’re really congested
Johnae Council December 13, 2025 AT 05:26
Dave’s “try saline first” line sounds nice but honestly most people will just reach for the drug anyway-why not just admit the spray is the real go‑to and the guidelines are just red tape?
Erin Leach December 20, 2025 AT 04:06
I hear you, but many pregnant folks are scared of anything that isn’t 100 % proven. A gentle reminder that talking to a midwife can clear up a lot of that anxiety might help
Brady Johnson December 27, 2025 AT 02:46
Laura, your sarcasm is cute until you realize some moms actually need that quick relief and your flippant tone just fuels the stigma around taking any medication while pregnant
Nic Floyd January 3, 2026 AT 01:26
Oxymetazoline is a topical alpha‑adrenergic agonist that induces vasoconstriction in the nasal mucosa. Its mechanism of action involves binding to α1 receptors on vascular smooth muscle cells leading to reduced blood flow and edema. The pharmacokinetic profile shows minimal systemic absorption due to the limited mucosal surface area. Plasma concentrations after a standard dose remain well below the threshold associated with systemic effects. In pregnant physiology the increased blood volume and renal clearance could theoretically alter drug disposition but the low baseline absorption mitigates this impact. Regulatory bodies such as the FDA and EMA have assigned a narrative risk label reflecting insufficient human data. Category C classification indicates animal studies have shown some adverse outcomes at high doses without robust clinical trials. Clinical guidelines therefore advise limiting use to three consecutive days to avoid rhinitis medicamentosa. Saline irrigation is recommended as first‑line therapy because it presents zero pharmacological risk. If decongestion is essential a single dose of oxymetazoline can be considered after evaluating the benefit‑risk ratio. Recent obstetric literature suggests that short‑term exposure does not correlate with congenital anomalies in epidemiological cohorts. However, the lack of large‑scale prospective studies means that absolute safety cannot be guaranteed. Counseling patients involves discussing the theoretical risk of uterine vasoconstriction versus the symptomatic relief provided. Healthcare providers often document informed consent when prescribing topical decongestants during pregnancy. Ultimately the decision should be individualized and informed by both clinical judgment and patient preference 😊